compare the element
All known substances can be classified as solids,
liquids, gases, or plasma. In addition, a fifth state of matter, the Bose-Einstein
condensate has been discovered recently. However, it is not stable at normal
earth conditions. Likewise, although plasma is the most abundant state of
matter in the Universe, it is not common on the Earth under normal
conditions, except for lightning. Most matter that students are familiar
with will therefore be in a solid, liquid, or gaseous state.
An element is a pure substance that cannot be decomposed into simpler substances by normal chemical means. There are 109 different elements. Ninety of these are naturally occurring; the rest have been created in laboratories. Elements 110 and 118 are still being researched on. There will be more elements as technology can identify them. A symbol is used to represent the full name of an element. For example, H represents hydrogen; O represents oxygen, and Al represents aluminum. Sometimes the Latin name for an element is used as the basis for its symbol, for instance K represents potassium (kalium in Latin).
Three subatomic particles compose elements: protons, neutrons, and electrons. Protons, which have an electrical charge of +1, and neutrons, which have a neutral charge, make up the nucleus of an element. This nucleus is surrounded by a "cloud" of electrons, each of which as a charge of -1. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits can contain a set number of electrons. For instance, the first orbital from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Each shell may not be full, depending on the number of electrons in the element, and the inner shells fill before the outer shells fill. Sodium, for example, has 11 electrons, which are located in the first, second, and third shells (2+8+1.)
An element has a uniform composition. Different elements may join together; these combinations are called compounds. A compound can be separated into its component elements by chemical means. For example, common table salt is a compound made of two elements: sodium and chlorine. Table salt can be broken down into sodium and chlorine by mixing it with water. However, sodium and chlorine cannot be easily broken down into any simpler forms.
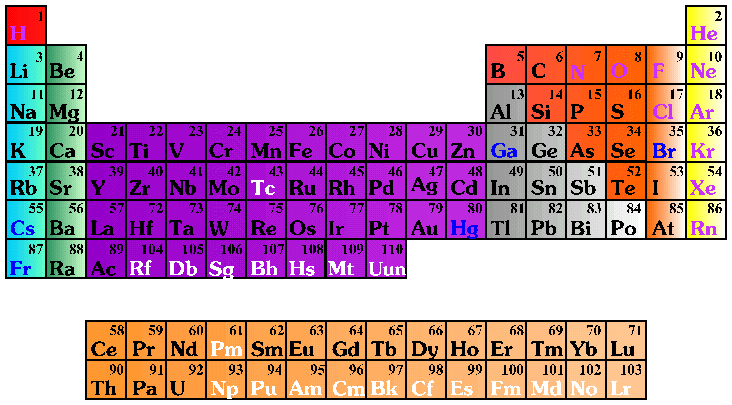
An element is a pure substance that cannot be decomposed into simpler substances by normal chemical means. There are 109 different elements. Ninety of these are naturally occurring; the rest have been created in laboratories. Elements 110 and 118 are still being researched on. There will be more elements as technology can identify them. A symbol is used to represent the full name of an element. For example, H represents hydrogen; O represents oxygen, and Al represents aluminum. Sometimes the Latin name for an element is used as the basis for its symbol, for instance K represents potassium (kalium in Latin).
Three subatomic particles compose elements: protons, neutrons, and electrons. Protons, which have an electrical charge of +1, and neutrons, which have a neutral charge, make up the nucleus of an element. This nucleus is surrounded by a "cloud" of electrons, each of which as a charge of -1. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits can contain a set number of electrons. For instance, the first orbital from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Each shell may not be full, depending on the number of electrons in the element, and the inner shells fill before the outer shells fill. Sodium, for example, has 11 electrons, which are located in the first, second, and third shells (2+8+1.)
An element has a uniform composition. Different elements may join together; these combinations are called compounds. A compound can be separated into its component elements by chemical means. For example, common table salt is a compound made of two elements: sodium and chlorine. Table salt can be broken down into sodium and chlorine by mixing it with water. However, sodium and chlorine cannot be easily broken down into any simpler forms.
PROCEDURE:
-
Discuss the properties of elements with the students. Review the structure of the periodic table. Ask students questions about the different elements and see if they can locate them on the periodic table.
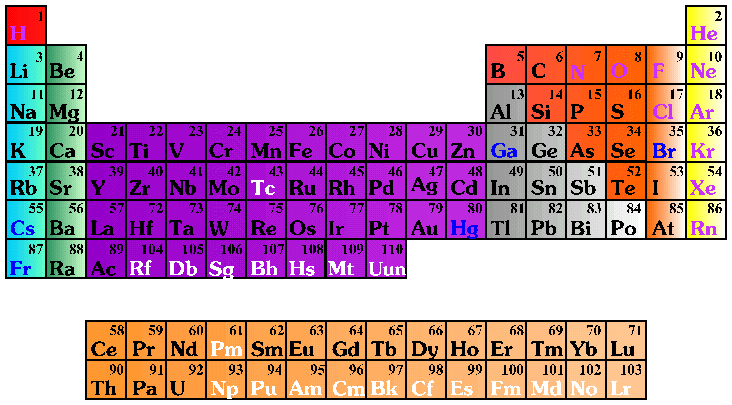
- Use the Periodic Table placemats to explore elements with the students. When they examine the chart, the students may ask the meaning of the numbers surrounding the element symbols. The number in the upper left corner is the atomic number, i.e., the number of protons inside the nucleus of the element. The number in the lower left is the atomic mass or atomic weight, which is essentially a measurement of how heavy the element is.
Explain the basic subatomic structure of elements. Tell the students that protons and neutrons reside inside the nucleus. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits represents a set number of electrons. For instance, the first orbit from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Sodium for instance, has 11 electrons located in the first, second, and third shells (2+8+1.)


BalasHapusMagnesium has atomic number 12 and mass number 25. The number of electrons in magnesium ion is ...
OK mega
HapusThe most common magnesium ion has a +2 charge
ok mega,,
BalasHapusAn ion forms when an atom either loses or gains electrons. If the atom gains electrons, it becomes a negatively charged ion. If it loses electrons, it becomes a positively charged ion. In the case of magnesium, the most common ion is Mg+2. This occurs when magnesium hydroxide, the main ingredient in Milk of Magnesia, forms. It is possible for magnesium to form bonds that create a different ion.
How to distinguish an element if unknown substance contained therein
BalasHapusChromatography: Going Mobile
HapusChromatography is used by basically everybody when doing experiments. Of course, that’s actually not a very clear statement, given that there are about a billion different types of chromatography, but technically true.
Chromatography is used with simple mixtures of compounds to determine what’s in there. Because it’s usually the case that you have some idea what these compounds are, you can figure out the identities of these compounds using this method. Which is vague, so I’ll just explain it:
The basic idea behind chromatography is this:
You’ve got a tube of some kind that’s filled with stuff.³ The stuff inside the tube just stays inside the tube, so it’s called the “stationary phase.”
Either a liquid or gas is pumped into one side of the tube. These are called either the “carrier liquid” or “carrier gas”, and the flowing stuff consists of the “mobile phase.”
At a time referred to, conveniently enough, as the start time (T = 0), the mixture of interest is injected into the front of the tube.⁴
As the different components in the mixture travel through the tube, they alternately stick to the stuff in the stationary phase and then rejoin the mobile phase. If these particles are strongly attracted to the stationary phase, they’ll take longer to come out of the tube than if they were weakly attracted.⁵
By measuring the amount of time it takes for each component to come out the other end of the tube, you can figure out what it is.
Please give examples about compound ! Thanks
BalasHapusOK AYU
HapusExamples of Compounds
Examples of Compounds - Common Compounds
There are millions of compounds. The following Examples of Compounds shows some of the most common compounds and their chemical formulas or equations.
Examples of Compounds
Examples of Formulas for compounds Examples of names of
common compounds
H2O
Water
C6H12O6
Glucose
C2H6O Alcohol
NaCl
Salt
C2H6O
Ethanol
C2H4O2 Vinegar
NH3
Ammonia
C2H4O2
Acetic Acid
C4H10
Butane
H2SO4
Sulfuric Acid
CH4
Methane
C12H22O11
Sucrose
C3H8
Propane
NaHCO3
Baking Soda
N2O
Nitrogen
C6H8O7
Citric Acid
C8H18
Octane
C10H16O
Camphor
Examples of Formulas for compounds Examples of names of
common compounds
wht is the elements?
BalasHapusAn element is a pure substance that cannot be decomposed into simpler substances by normal chemical means. There are 109 different elements. Ninety of these are naturally occurring; the rest have been created in laboratories. Elements 110 and 118 are still being researched on. There will be more elements as technology can identify them. A symbol is used to represent the full name of an element. For example, H represents hydrogen; O represents oxygen, and Al represents aluminum. Sometimes the Latin name for an element is used as the basis for its symbol, for instance K represents potassium (kalium in Latin).
Hapus